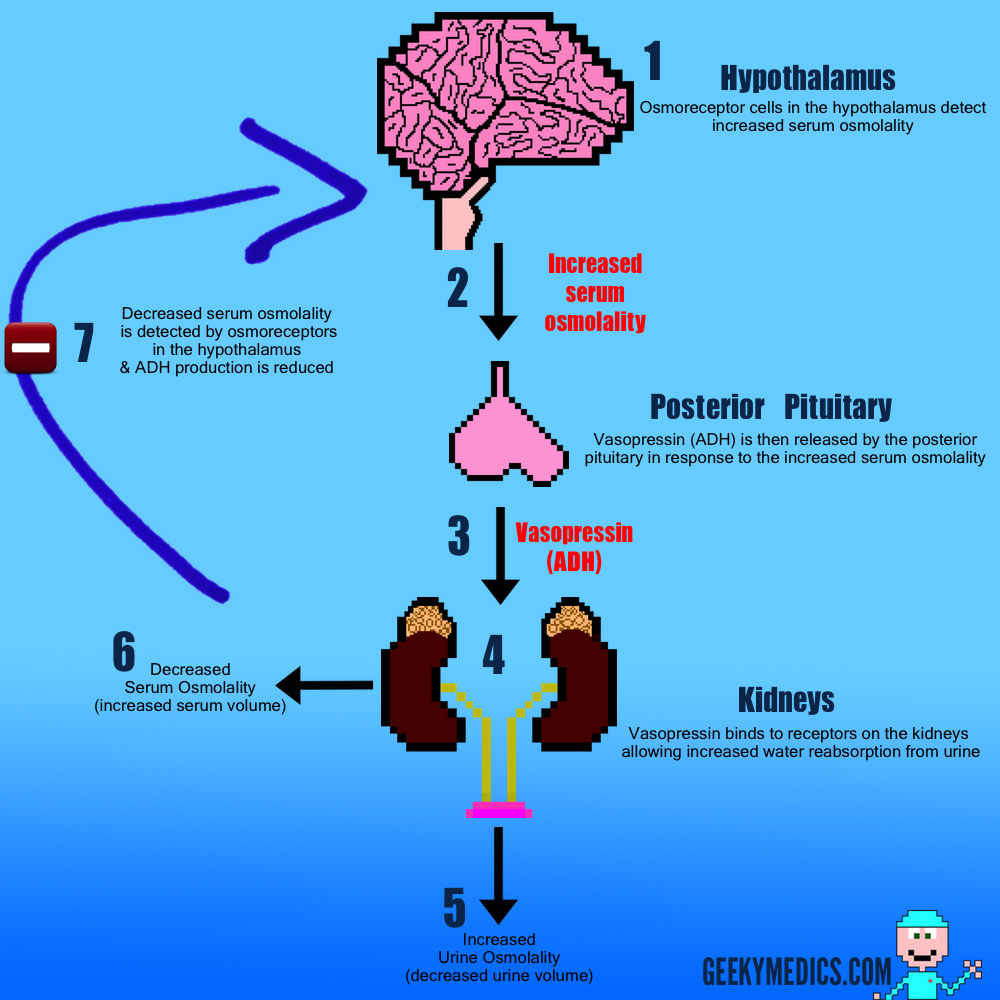
What is Type 1 Diabetes Mellitus?
Type 1 Diabetes Mellitus is a form of diabetes that occurs due to autoimmune destruction of the insulin producing pancreatic beta cells. The loss of insulin producing cells leads to insulin deficiency which in turn causes hyperglycaemia. Patients with this disease will always require insulin to control blood glucose levels. Without treatment patients develop diabetic ketoacidosis which can progress to coma and death. It is therefore essential to recognise signs of type 1 diabetes as early as possible to allow prompt treatment.
The disease commonly affects young otherwise healthy individuals however it can occur at any age. The disease has been associated with other autoimmune diseases such as thyroid disease, coeliac’s disease, pernicious anaemia, vitiligo and others. It is thought to be the result of a genetic susceptibility combined with exposure to an environmental trigger (e.g. virus)
Causes
Genetic
Over 90% carry HLA DR3 +/- DR4 suggesting these genes add significant risk of disease
The gene IDDM1 is thought to be strongly associated with development of type 1 diabetes
Environmental
Studies of identical twins have shown only 30% concordance of developing type 1 diabetes.
This suggests that the environment plays a very important role in the development of the disease
It has been suggested that a virus may trigger the disease in genetically suceptible individuals
This is thought to occur due to a virus having similar antigens to the pancreatic beta cells causing inappropriate immune activation. The Coxsackie virus is one organism which has been implicated as a trigger in some cases
Symptoms & Signs
Symptoms
Polyuria -frequent urination
Polydipsia -increased thirst
Polyphagia – increased hunger
Weight loss - unrelated to diet
Lethargy
Genital thrush
Dehydration
Blurred Vision
Nausea
Abdominal Pain
Signs
Hyperglycaemia
Glucosuria - glucose in urine
Ketone bodies
Diabetic retinopathy
Peripheral Neuropathy
Foot ulcers
Pear drop scented breath -ketoacidosis
Diabetic Ketoacidosis
Diabetic Ketoacidosis (DKA) is a potentially life threatening condition most often seen in type 1 diabetics. It occurs due to a lack of insulin, which prevents the bodies cells from absorbing and utilising any glucose in the blood. As a result the body switches it’s metabolism to breakdown fat to produce energy. This process produces acidic ketones as a byproduct and these build up in the blood causing a metabolic acidosis. Left untreated the condition ultimately leads to coma and death, therefore signs need to be recognised early and treated quickly.
Clinical features
Nausea/Vomiting
Severe abdominal pain
Dehydration
Breath smells of pear drops
High Ketones
Severe Hyperglycaemia
Severe Hyperkalaemia
Kussmaul Respiration - deep gasping breathing
Cerebral Oedema
Coma
Treatment
The main aims of treatment are to reduce blood glucose & ketone levels using insulin whilst correcting dehydration & other electrolyte abnormalities:
- Insulin
- Potassium
- Bicarbonate
- IV Saline
Investigations
Diagnosis is made based upon:
- Classical type 1 diabetes symptoms – weight loss, ketoacidosis, polyuria, polydipsea etc
- Hyperglycaemia - (fasting glucose >7 mmol/L) or (random glucose >11.1 mmol/L)
.
Ketones - can be checked to assess if patient has ketoacidosis
Antibodies – shouldn’t be used for diagnosis but may be helpful in differentiating type 1 & type 2
Management
All patients with type 1 diabetes require insulin replacement therapy
Patients must regularly check their blood glucose levels & adjust their doses accordingly
Subcutaneous Insulins
Very Fast Acting – usually injected at start of meal to match what is eaten – e.g.Novorapid
Combination Insulin - Fast & long acting insulin mixed together - e.g. Novomix 30% fast 70% long
Long acting - acts over long period of time therefore good for night time - e.g. Glargine
Regimes
The regime of treatment is influenced by the type of lifestyle an individual has:
- Biphasic regime – twice daily combination insulin -useful if patient has a predictable lifestyle
- QDS regime – use Novorapid before meals & have 1 dose of long acting insulin at night – flexible
Insulin Pump Therapy
Insulin pump therapy aims to more closely mimic the function of a healthy pancreas
The pump uses fast acting insulin such as Novorapid
It involves having a small cannula placed in the subcutaneous fat, usually around the waist
This is connected to the insulin pump which provides the insulin infusion & boluses
.How it works
The pump releases a constant rate of insulin throughout a 24hr day, known as the basal rate
This can be altered in real time by the patient depending on their needs – 50% increase when ill
.The pump can also give a bolus of insulin before each meal, this involves:
- Patient calculating the amount of carbohydrates in a meal
- Then calculating amount of insulin required depending on their insulin:carb ratio
- Setting the pump to provide the required units of insulin over a short time period e.g. 15 mins
Insulin pumps give individuals much more precise control over their blood glucose level.
This is empowering for the patient & over time they learn which settings work best for them
Insulin pumps have been shown to improve quality of life & lower HBA1C if used appropriately
There is an increased risk of DKA due to using only rapidly acting insulin
.NICE recommends insulin pump therapy for type 1 diabetes patients who:
- Suffer repeated & unpredictable episodes of severe hypoglycaemia when trying to reach acceptable HBA1C
- HBA1C levels have remained above 8.5% with multiple daily injections
- In children less than 12 years where multiple daily injections is impractical or innappropriate
Eye Screening
There is now an annual national eye screening program for all people with diabetes
This aims to recognise and treat diabetic retinopathy at an early stage before vision is lost
Podiatry
Its essential for those with diabetes to go to regular appointments with a Podiatrist
Diabetes can cause peripheral neuropathy & vascular insufficiency which can lead to ulcers
The aim is to detect early signs of foot disease and take measures to prevent progression
This involves advice on foot care and appropriate foot wear among other things
HBA1C monitoring
The patient will have their HBA1C monitored by their doctor
The HBA1C shows the level of blood glucose control over the previous 3 months
It can identify patients with poor control and whose treatments may have become ineffective
It is normally recommended that a patient keep their HBA1C below 7.5%
Prognosis
The longer an individual has diabetes the more risk they have of developing complications:
- Peripheral Neuropathy
- Diabetic Retinopathy
- Chronic Renal Failure
- Cardiovascular disease - MI
The risk of developing these complications can be largely reduced by good blood glucose control. Therefore the probability of each of these depends on the individual patient & co-morbidities